Abstract
Introduction: Burkitt and Burkitt-like lymphoma (BL/BLL) are highly proliferative germinal or post-germinal B cell tumors. Few studies have evaluated the impact of autologous stem cell transplantation (ASCT) on disease outcomes. We performed a systematic review to analyze the efficacy of ASCT in upfront consolidation and for treatment of relapsed/ refractory cases in adult BL/BLL.
Methods: A systematic search of the electronic database (PubMed, Cochrane, Google Scholar, and EMBASE) was conducted for relevant studies. Any clinical trial, prospective study, or retrospective study with clear outcome measures on the efficacy of ASCT in adult patients with BL/BLL were eligible for inclusion. The overall survival (OS), progression-free survival (PFS), complete response (CR), partial response (PR), and progression or relapse were used to assess the efficacy.
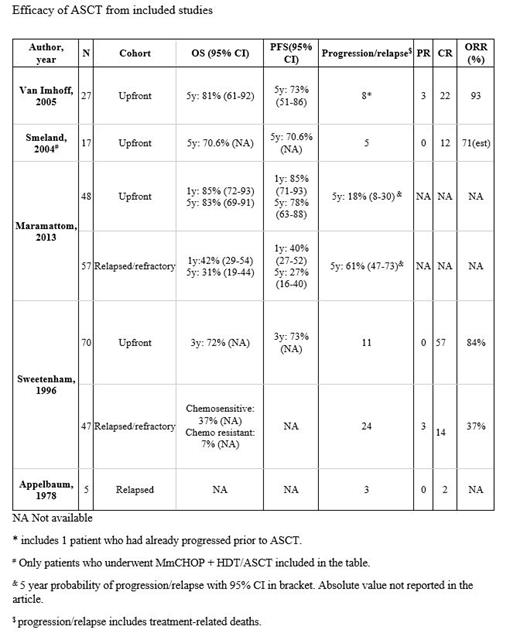
Results: For patients who underwent ASCT in first CR, 5-year PFS and OS ranged between 70-78% and 70-83%, respectively. For relapsed/refractory disease, 5-year PFS and OS were reduced at 27% and 31%, respectively. Patients undergoing ASCT for chemoresistant disease fared poorly with an OS of 7% at 3 years versus 37% for chemosensitive disease (p ≤ 0.00001). The overall response rate (CR or PR) to ASCT for patients transplanted in first CR ranged between 71% and 92%. This was reduced to 37% for patients who were transplanted in disease status other than first CR. Disease progression/relapse was observed in 16% to 29% of the patients transplanted in first CR, and 55% to 60% in relapsed disease.
Conclusion: Our systematic review found insufficient evidence to support ASCT over chemotherapy alone in the first remission for adult BL/BLL. Evidence supports guidelines recommending ASCT for chemosensitive disease but suggests there is no benefit to ASCT for chemoresistant disease. Prospective studies of ASCT are required to definitively understand the value of ASCT for BL/BLL.
Bhatt: Pfizer: Research Funding; Tolero Pharmaceuticals, Inc: Research Funding; Incyte: Consultancy, Research Funding; Jazz: Research Funding; Abbvie: Consultancy, Research Funding; Partnership for health analytic research, LLC: Consultancy; Servier Pharmaceuticals LLC: Consultancy; Rigel: Consultancy; National Marrow Donor Program: Research Funding; Abbvie: Consultancy, Research Funding; Genentech: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal